Standing the Test of Time
A deep dive into the factors that define a wine's shelf life and its ability to age gracefully despite the inevitable effects of oxygenation.
The notion of an expiration date for wine is a silly one. Though it is food—a consumable good found on supermarket shelves the world over—there is general acceptance of the fact that its lifespan is measured in years, and that in any case no pathogens will develop given wine’s relatively high alcohol content. That said, there is a clear qualitative difference between a wine drunk in its prime, and one which has been left for too long or stored improperly.
Look at any serious review of a fine wine and you'll likely find the critic giving the wine a "when to drink" window, during which your enjoyment should be optimal. For example, with regard to the highly rarefied—and rather pricey—2014 Domaine des Comtes Lafon Montrachet Grand Cru, well-renowned French critic duo Bettane et Desseauve suggest it should be consumed at will between the years 2017 and 2034. Robert Parker's The Wine Advocate, meanwhile, suggests consuming this gem between 2023 and 2050 for an optimal experience. Though naturally based on extensive experience with this and other wines of a similar calibre, these time frames would appear to reflect the tastes and subjective views and preferences of the critic as much as the intrinsic qualities of the wine itself. As a side note, if any of you reading this happen to be critics yourselves, I'd be very interested to hear your thought process when deciding on how to advise consumers of when to drink a particular wine.
Of course, at £3,840 ($5,117) per bottle (according to data from Wine Searcher at the time of writing), the above example can't exactly be said to be reflective of your average wine. In contrast to the expected 20–36 year lifespan (depending on your preferred guru) for the 2014 Grand Cru, most white wines tend to have a recommended drinking window within 2 to 3 years of the vintage date. Even this, however, is nothing but a guesstimate, and there are stories of supermarket gems maintaining their freshness after decades of appropriate storage.
What I want to look at in some more detail in this article is what is in a wine that can allow it to stand up to extended storage, and take a look at an intriguing new study that may aid in removing some of the guesswork when establishing these drinking windows. To avoid making the topic overly complicated, and to be in line with the study mentioned above, we'll be focusing on white wines, which at any rate are often more susceptible to the ravages of time given their lack of tannins. This will get a bit technical, but with a complicated drink like wine, some talk of chemistry is needed to actually understand what goes on in a bottle, so bear with me.
What Determines the Ageing Potential of a Wine?
The ageing potential of a white wine is principally governed by its intrinsic structural and chemical composition—those qualities imparted by the grape variety, vineyard site, vintage conditions, and winemaking decisions. At the heart of age-worthiness lies balance, and it is the interplay of acidity, phenolic compounds, sugar, alcohol, and residual sulphur dioxide which separates the merely good from the truly exceptional wines out there. Among these variables, acidity is paramount. High acidity acts as a natural preservative, helping the wine retain freshness over time and allowing it to evolve rather than simply deteriorate. Grape varieties such as Riesling, Chenin Blanc, and Semillon are renowned for their longevity largely due to their high natural acidity and, in certain styles, moderate alcohol and elevated dry extract, which provide structural resilience. A key measure of this acidity is pH; wines with a low pH (typically between 2.9 and 3.3) are chemically better equipped to withstand the test of time.
The chemical advantages of a low pH are manifold. Most notably, it serves to inhibit oxidative reactions, which degrade aromatic and flavour compounds over time. In a more acidic environment, phenolic compounds are less likely to undergo polymerisation through oxidation, which helps preserve the wine’s clarity and aromatic precision. In addition, low pH increases the effectiveness of sulphur dioxide (SO₂), a widely used antioxidant and antimicrobial agent in winemaking. The proportion of molecular, or free, SO₂—the most active and protective form—is significantly higher at lower pH levels. This means less SO₂ is needed to achieve the same protective effect, which in turn helps maintain the delicacy of a wine’s aroma profile. A low pH also acts as a formidable barrier to spoilage organisms such as Brettanomyces, Lactobacillus, and Acetobacter, all of which struggle to thrive in more acidic conditions. Taken together, these factors mean that low pH wines are more stable, more resistant to spoilage, and better suited to graceful ageing.
Beyond acidity, certain white wines benefit from phenolic structure, either derived from oxidative winemaking styles—as in Savagnin from the Jura—or from extended skin contact, as is the case with orange wines. Phenolic compounds, while less abundant in whites than in reds, can still lend structural grip and oxidative resistance. Sugar, too, plays a role: wines with some residual sugar, such as German Prädikatsweine or Sauternes, possess higher osmotic pressure, which inhibits microbial activity and slows oxidation. Additionally, some grape varieties contain aromatic precursors that slowly hydrolyse over time, releasing complex compounds such as terpenes or norisoprenoids, which contribute to the layered, tertiary aromas characteristic of well-aged whites. When all these elements—acidity, phenolics, sugar, and aromatic potential—are held in equilibrium, a white wine is not only capable of enduring the years, but of transforming into something more intricate and profound with age.
Whether this is desirable is of course a different story. Some prefer the crisp, fruit-forward aromas of a young wine, while others relish the velvety elegance of an aged wine full of tertiary aromas like nuts and mushrooms. Personally, I think a well-aged Chardonnay—the 2013 Domaine Roulot springs to mind—represents one of the finest wine-drinking experiences money (quite a bit of it, unfortunately) can buy.
While the above talk of the chemical constituents of a wine might sound a bit clinical, the mystique of a truly spectacular wine remains alive in the sheer complexity of factors that have to come together for that perfect balance to be achieved. Though outside the scope of this article, it depends both on the terroir the wine is grown in—microbiology and soil health inclusive—and the winemaking protocols applied to the resulting juice.
A Formulaic Approach
Through testing, any juice or wine can be thoroughly analysed to determine its acidity, concentration of phenolic compounds, sugar, alcohol and of course SO₂. The latter is particularly of consequence due to the fact that, as mentioned above, it is one of the most commonly used antioxidant and antimicrobial agents in winemaking. While also a natural by-product of yeast, sulphites must be one of the most poorly understood, yet most talked about additives in winemaking, being the focus of what might be called the "no added sulphites" movement. Disregarding irrational concerns about the safety of sulphites and their purported link to the dreaded hangover (look up the concentrations in dried fruits and tell me you’ve ever suffered from a papaya-induced headache…), its role as a stabiliser and preservative is absolutely crucial to the longevity of a wine. While it is true that natural wines with low or no added sulphites may, under the right conditions, age gracefully as well, some SO₂ is very helpful in preventing oxidation, which is one of the main causes for wine’s gradual deterioration over time.
A recent article by Yingxin Miao and Andrew L. Waterhouse, published in the American Journal of Enology and Viticulture, titled Rapid White Wine Shelf-Life Prediction by Forecasting Free SO₂ Loss Post-Bottling sheds some intriguing insights that may provide winemakers with a valuable tool to estimate how long their wines will be able to withstand the slow but steady oxidation that occurs once a wine is in the bottle.
While reading the study in full is recommended, I’ll do my best to explain the core findings in what follows.
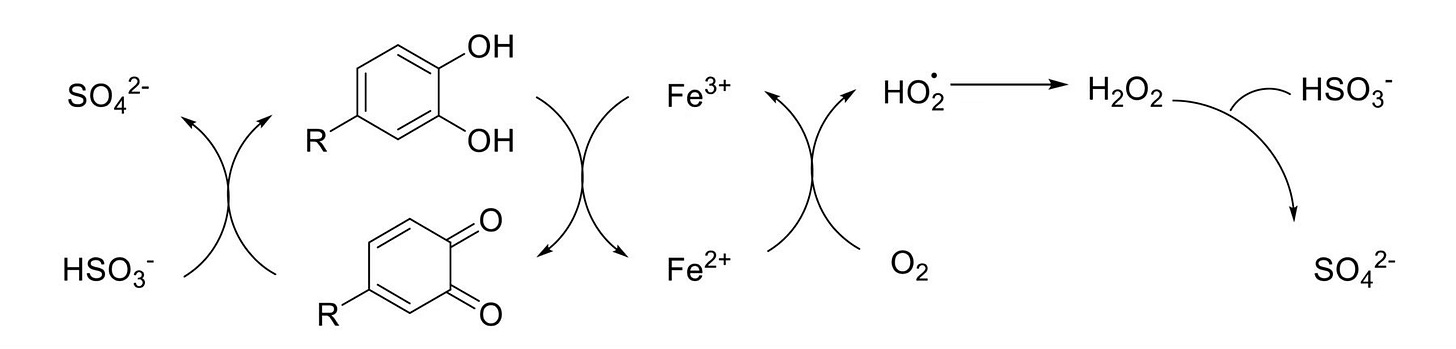
First off, compared to other antioxidants found in wine, SO₂ is typically present at relatively high levels, with a mean concentration of free SO₂ in white wine of 26 mg/L and total SO₂ of 130 mg/L. There are two primary mechanisms in which SO₂ acts as an antioxidant. Firstly, when wine is exposed to oxygen, SO₂ is involved in the recycling of phenolics from quinones, which prevents an accumulation of quinones which in turn prevents browning. The chemical reaction is a bit fancy, but in short, quinones are reactive oxidation products formed when phenolic compounds in the wine (like catechins or caffeic acid) interact with oxygen. They are what is known as electrophilic and can react with nucleophilic wine components, damaging aroma-active thiols or other sensitive compounds. However, antioxidants such as glutathione, ascorbic acid, and sulphur dioxide can reduce quinones back to their original phenolic forms—effectively recycling them.
Technically, this involves a redox cycle: quinones are reduced to hydroquinones or back to phenols, halting oxidative chain reactions and preserving wine stability and freshness. All in all, very neat—and a good reason to not necessarily shy away from all SO₂ usage like some insist on doing.

The second mechanism which SO₂ is involved in helps control the aromas associated with oxidation. In particular, SO₂ binds with various aldehydes in wine, which are largely by-products of the fermentation process. However, when wine oxidises, ethanol can be converted into acetaldehyde, a volatile compound that contributes bruised apple or sherry-like aromas if it accumulates. To some, a small concentration of this can be appealing, but in excess it is a pretty well-recognised fault and the wine would be deemed oxidised. However, sulphur dioxide reacts readily with acetaldehyde, forming what is known as a bound, non-volatile complex. Unlike the above-mentioned redox cycle, this process is non-reversible, tying up free SO₂ in the process. That said, not all of these aldehyde bonds are of equal strength and, over time, as the free form of SO₂ is being depleted, the weakly bound SO₂ slowly dissociates and replenishes the free SO₂ pool. This exchange allows for a ratio to be calculated between the change of total SO₂ and the change in free SO₂ during oxidation. This represents what is known as the wine’s sulphite buffering capacity. Over time, this capacity is gradually reduced as more and more free SO₂ is tied up in strong, non-reversible bonds. The study references another paper by Sacks et al., 2020, which reported that there is no buffering when free SO₂ is <5 mg/L. As the amount of free SO₂ is reduced, free aldehyde concentrations are able to increase, as they are no longer binding with the sulphites. A paper by Godden et al., 2001, reports that the detection threshold for aldehydes occurs when free SO₂ is <10 mg/L.
To recap the above in two quick sentences, the two antioxidative mechanisms in which SO₂ plays a role are what allow wines to keep their colour and prevent excessive concentrations of unpleasant aldehyde aroma compounds from accumulating. Over time, free SO₂ is reduced as it gets tied up in strong aldehyde bonds, eventually dropping to a point where aldehydes can accumulate to a noticeable level.
The hypothesis for the study by Miao and Waterhouse is based on the notion that if you could project this decline in free SO₂, you could predict a wine’s shelf life. To test this, they subjected eight wines to a rapid ageing process, involving saturating the wine with oxygen and heating it to 45°C. While they acknowledge that there may be confounding variables at play under regular storage conditions, the main difference caused by this method is the speed of the chemical reactions at play. This does tie in to my earlier article about wine storage, which I’ll link to below for those interested.
Now, there is much more to this paper, and I urge those of you so inclined to go directly to the source. But in short, the results of the study found that the SO₂ depletion ratio can be expressed as a constant of 1.8998. When entered into the equation below—alongside the free SO₂ in the bottle and the oxygen transmission rate of the closure—this constant can be used to predict the time before aldehyde concentrations reach detectable levels, marking the effective shelf life of the wine.
Potential wine shelf-life = Aging time = Free SO2 in bottle − Minimum free SO2 after aging (10 mg/L) / Free SO2 depletion factor × O2 transmission rate
The practical example of the above formula used in the paper is as follows: assuming an initial free SO₂ level in bottle of 28 mg/L, an O₂ transmission rate of the bottle closure of 3 mg/year, and a minimum SO₂ level of 10 mg/L, the potential shelf life of the wine in question is 3.16 years.
If this study can be corroborated and the free SO₂ depletion factor can be shown to in fact be constant, I think that is pretty revolutionary, giving winemakers who care to do the necessary tests a far better understanding of the ageing potential of their wine.
As discussed, however, sulphites are not the only contributor to a wine’s ability to age gracefully, and the above study only targets a relatively small part of the immensely complex liquid that is wine.


Final Thoughts
If you’ve made it this far, well done. It’s a lot to take in, and as those who have had a look at the link to the study I reference above will see, I have completely glossed over the discussion of the SO₂:O₂ molar reaction ratio, which plays a critical role in how the oxygen in the wine reacts to the free SO₂ in the liquid. In part, this is because I would have to delve far deeper into the broader topic of chemistry in order for it to make sense to me—let alone for me to write about it with any authority.
There’s also the more practical question of “how can I as a consumer benefit from this knowledge?”, which I’m sure some of you might be thinking. Primarily, I hope that this gives a bit of a broader context to the whole sulphites debate, while shedding light on what actually goes on when a wine ages. We (us wine nerds who bother thinking of this sort of stuff) know that wines develop over time and will have seen talk of, or experienced, wines that are deemed to be “past their prime” or plainly suffering from oxidative faults, yet the dynamics of what goes on with the liquid rarely get discussed. We talk of consequences and prevention strategies without necessarily grasping the connecting part which explains how the consequences (faulty wine) come about. At least not beyond the surface level, which I tend to find unsatisfactorily basic. That it is also not just about sulphites, but about how those sulphites interact with the wine’s acidity and sugar levels, has been pretty eye-opening for me—connecting dots I hadn’t connected until researching this article. For instance, I’ve been aware that wines like Riesling can age well, but I have gained a better appreciation for just why that is.
Hope you have enjoyed the above as much as I have done researching it, and would love to hear your thoughts on it.







"When all these elements—acidity, phenolics, sugar, and aromatic potential—are held in equilibrium, a white wine is not only capable of enduring the years, but of transforming into something more intricate and profound with age."
Chateauneuf du Pape Blanc!
"Natural wines with low or no added sulphites may, under the right conditions, age gracefully as well, some SO₂ is very helpful in preventing oxidation, which is one of the main causes for wine’s gradual deterioration over time."
When you've paid $50 a bottle for a natural wine whose structure disintegrates within 30 minutes of popping the cork (all fruit gone, brett city), you will think two or three times about spending that much money on natural wine again.
Great article, thank you. The mechanisms of SO2 are fascinating.